Abstract
Background: myelofibrosis is a rare hematological malignancy which can be cured by hematopoietic stem cell transplant (HSCT) and many studies are showing that 3 to 5-year survival after HSCT is between 45 to 70% in relative standard condition (chronic phase disease, HLA matched donor). Relapse and transplant-related mortality are more frequent the first 2 years post HSCT. To optimize the long-term management of these patients, we raised the question of long-term outcome in patients surviving in remission at least 2 years after HSCT. Furthermore, that's a recurrent question from the patient to know if his life expectancy can reach the general population one many years after transplant.
Method: from the EBMT registry, patients transplanted from 1995 to 2014 with a diagnosis of primary or secondary myelofibrosis even if the disease was transformed into acute myeloid leukemia (AML) at any point before the transplant were included. Only countries performing at least 20 transplants for this indication during the period were included. Among the 2461 patients from 15 countries fulfilling these criteria, 1245 and 650 were alive at 2 and 5 years, respectively. Overall survival was compared to an age, sex, year and country matched population. Excess mortality was defined as the difference between the observed mortality in the myelofibrosis patients and the mortality in a matched population and represents mortality specifically due to myelofibrosis and its treatment. Multivariable cox models for excess mortality were calculated for all patients, patients alive at the 2 and at the 5 year landmarks after transplant.
Results: median age at time of transplant was 55 years (from 19 to 76 years); 1906 (77%) patients had primary myelofibrosis, 421 (17%) secondary myelofibrosis and 134 (6%) were transformed into acute myeloid leukemia (AML) at time of transplant. A reduced intensity conditioning regimen was used in 1503 (63%) patients and the majority of patients (87%) received peripheral blood stem cell transplant. 1024 (43%) patients were transplanted from an HLA-matched sibling donor. The majority of events (death, relapse) occurred the first 2 years of transplant. Two-year cumulative incidence of relapse and non-relapse mortality was 23% (95%CI: 21-25%) and 26% (95%CI: 25-28%). The multivariable Cox model for the excess mortality for the whole cohort (without landmark) finds that myelofibrosis transformed into AML (HR: 1.7, p<0.0001), irradiation in regimen (HR 1.3, p=0.002), the use of a non-HLA matched sibling donor (HR 1.3, p<0.0001) and the age (p<0.0001) were all significantly associated with a worse survival. On the other hand, female gender was associated with an improved outcome (HR 0.9, p=0.055).
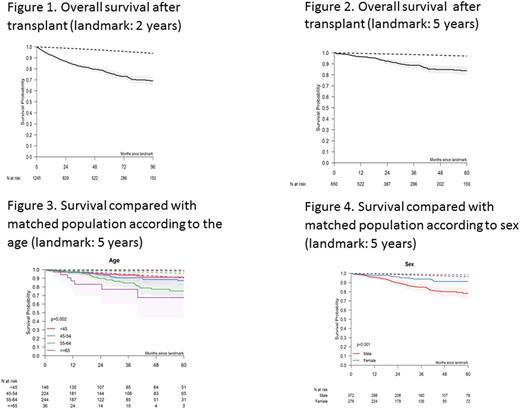
We also analyzed long-term outcome after the 2 and 5 year landmarks. Survival at 8 years for patients alive 2 years after transplant was 69% representing an excess of mortality as compared to the matched population (Figure 1). 5-year survival for patients alive 5 years after transplant was 84% (Figure 2). With the 5-year landmark, cumulative incidence of relapse was 13% and non-relapse mortality was 8% 5 years later. Regarding primary causes of deaths with the 5-year landmark, there were 24 relapses/progressions from the primary disease, 13 other malignancies, 12 GVHD, 13 infections, 1 toxicity and 17 unknown causes. In patients alive at the 2 year landmark, female gender was strongly associated with a better outcome (HR 0.7, p=0.018), as well as the advancing age (HR 1.03, p<0.0001). None of the other risk factors had a significant effect in this landmark population. In patients alive at the 5 year landmark, gender (HR: 0.4, p=0.026) and age (HR: 1.04; p=0.028) were confirmed as associated independently with a better outcome (HR 0.44). Figures 3 and 4 show overall survival by age and recipient gender, compared to the respective matched populations.
Conclusion: long-term analysis in patients transplanted for myelofibrosis shows that there is still an excess mortality as compared to matched population but this excess of mortality is lower in younger patients and in women. Variables related to transplant procedures (type of donor, TBI) are no more risk factors for long-term outcome.
Schetelig: Sanofi Aventis: Consultancy, Research Funding; Roche: Honoraria; Abbvie: Honoraria; Janssen: Consultancy, Honoraria. Robinson: Riemser: Consultancy; Roche: Honoraria, Other: Travel Grants; Takeda: Consultancy, Honoraria, Other: Travel Grants; Gilead: Honoraria, Other: Travel Grants.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal